Adaptively Responsive Mutation on the Karyotypic Level Manifested as Pattern Differentiation and Morphogenesis in a Fungus: Further Steps into a New Paradigm, with Implications for Agriculture
I. Introduction: Environmentally Responsive Mutagenesis on Different Levels of the Genome
Relatively recent investigations of mutations generated in various unicellular or simple, undifferentiated colonial organisms have revealed what can be considered as the beginnings of a paradigm shift in biology. During the past 27 years, adaptively responsive, enhanced mutation has been found in bacteria and yeast, and in 1969, evident within the unicellular green alga, Chlamydomonas, an eukaryote. Though in the case of yeast, there was also a far earlier report (Lindegren, 1966) of adaptively responsive mutation. Such enhanced mutations, linked to stress, enabled the quick adaptation of the single cells of the organism to changed, stressful situations. In some studies, such adaptation to a particular nutritional stress enabled single cells in non-growing bacterial colonies to produce adapted, growing clones or sectors, called papillae. While in many other studies involving other types of nutritional stress, such adaptation enabled the growth of whole colonies from single cells during the stressful conditions. In 1989, 1990 and 1998, the author showed, through his own work on bacteria under nutritional stress, that the occurrence of adaptively responsive mutations resulting in growing colonies is under internal, genetic control or regulation, hence non-random, demonstrating developmental features. As pointed out, this suggested the evolution of an inner, mutator capacity that could have modulated evolution itself. Earlier in 1967, it was also pointed out by the author that the enhanced occurrence of many types of mutation was non-random, being under genetic regulation through internal mutator processes, whose existence in the past could have enhanced the degree of evolution from within. (Also, see references in Lieber, 2011 and references via http://www.googlescholar.com.
The non-randomness of enhanced mutations under stress to the organism has become clearly manifested repeatedly in the last 27 years of mutation research. However, during this 27-year period of investigations, the particular, non-random mutations studied were only adaptively responsive mutations to nutritional requirements and to the stress of antibiotics. And, in the case of the green alga, the adaptively responsive, frequent mutations occurring on the culture medium enabled growth of many colonies of joined cells in the presence of a growth inhibi11/17/2016tor in the culture medium, another type of chemical stress. These adaptively responsive, enhanced mutations enabled adaptation to stress on the biochemical or molecular level of organization in unicellular organisms, as opposed to higher levels of organization, such as on the level of morphogenesis in a multicellular, differentiated organism relatively far more complex than bacteria colonies and the colonial algae. In unique contrast, very frequent mutations at the chromosomal level due to or in response to physical stress can occur under inner control that lead to adaptive changes in differentiation of pattern and morphology in olive-green, multicellular fungal colonies having a central, crinkled morphology, sparsely populated with conidiophores/conidia, and of reduced growth rate. (See Lieber, 1998 for a description and earlier, relevant references.) An adaptively responsive, innerly-controlled, greatly enhanced mutation on a higher genomic level determining development was shown to exist many years previously to most of the mutation studies referred to above, with significant implications for evolution.
Investigation with the multicellular, differentiated eukaryotic fungus, Asperillus nidulans, an ascomycete, once considered a lower plant, revealed that very frequent mutations on the karyotypical or chromosomal level of organization were an adaptive response to high temperature stress. These environmentally responsive, adaptive, karyotypic mutations resulted in the production of many yellow sectors in each sparsely conidiated, olive-green colony. The sectors were composed of yellow, a-sexual reproductive structures, the conidiophores made up of yellow conidia-spores, the means of a-sexual production. The ensuing production or existence of such mutant sectors manifested itself phenotypically at a higher level as a new type of pattern-differentiation through such sectors and morphological change within fungal colonies (Lieber, 1972, 1976b, 1998.) Such a new pattern of differentiation and change in morphology, based on inner-controlled though environmentally responsive genomic changes, a responsive, inner-controlled hypermutation, were adaptive in the following ways: These genomic changes enabled, under temperature stress, major increases in vegetative, yellow spore production within differentiated, mutant yellow sectors and greatly increased growth rate of such, flat, yellow sectors of normal morphology. (The reader is directed to the photographs at the end of this article. Also, go to Figure 8.). This mutator system is in itself an example of a developmental system capable of further evolution through its own inner-controlled, adaptively, responsive mutability at the karyotypic level.
II. Details of the Investigation with Aspergillus nidulans, a Plant-Like Organism
The fungus investigated, Aspergillus nidilans, is a normally haploid, eukaryotic ascomycete with eight chromosomes. Its colonies have internally septate hyphae made up of multinucleated cells divided by the septae. Without chromosomal re-arrangements or new chromosomal configurations within the haploid genome, the fungus produces flat, grass green colonies due to green conidiophores emerging vertically from multinucleated hyphae composing the colonies. The colonies display high growth rates at various temperatures (Lieber, 1972, 1976a.). Colonies with a single, new chromosomal configuration in each of their nuclei have a crinkled morphology and a reduced growth rate, especially at high temperature. The new chromosomal configuration responds mutagenically to various temperatures (Lieber, 1976a.) This fungus produces a-sexually reproductive spores. Each spore, a conidium, has a single nucleus.
One particular strain of Aspergillus nidulans investigated at a high temperature had two chromosomal, non-uniform configurations in the haploid genome. These configurations have respectively partial duplications in trans of chromosomes I (Dp I) and III (Dp III). (These were carried on respective chromosomal translocations.) Aspergillus colonies with these two configurations in the haploid genome are much smaller than normal colonies. They display a crinkled morphology, especially pronounced at high temperature, and produce far less vegetative spores or conidia at higher temperature, e.g. 39.50 centigrade (Lieber, 1972, 1976b.) One of these configurations, referred to as Dp I, contains two genes for conidial or conidiophore color, one green and one for yellow. The two color alleles are heterozygous within the duplication, green being dominant to yellow, hence the green or olive- green color of the colonies, that is colonies having green or olive-green conidia and conidiophores within the crinkled area. In some nuclei, a specific region of Dp I containing the green allele is subject to deletions, resulting in yellow sectors of increased growth rate. The frequency of such deletion, and of corresponding yellow-sector production, is influenced by the other duplication, Dp III and temperature.
Modulated by temperature and the age of the conidia from which colonies are obtained [Lieber, 1972], Dp III controls the degree and pattern of deletion including the green allele on Dp I. As Dp III becomes reduced in size as a result of deletions having occurred from it, the reduced Dp III enhances the deletion of the genetic region including the green-allele region of Dp I. A deleted segment, a type of transposition element from Dp III probably inserted near the green allele on Dp I, may trigger, under the control of Dp III, such deletion. When this occurs under a temperature stress, that is a high temperature, this mutagenic, deletional interaction of the two configurations, via a likely transposition process, is enhanced to an even a far greater degree. Moreover, this mutagenic enhancement is clearly regulated, since the improved, yellow sectors, as a consequence of the deletions from Dp I in many nuclei, all emerge at the same time. Furthermore, this temporal control of deletion, clearly under the control of the reduced Dp III, becomes far more pronounced or effective at the stressful, higher temperature (Lieber, 1972, 1976b.) The dampening, epigenetic influence of age-affected conidia on the degree of mutagenetic interaction, in cultures obtained from these conidia, is also suppressed epigenetically through this higher temperature [Lieber, 1972].
Specifically, at that higher temperature, irrespective of the age-state of the conidia producing the fungal colonies, the adaptive consequences of this very frequent, deletional mutagenesis or instability, possibly during mitosis, on the karyotypic level of organization are fungal colonies that each symmetrically produce frequent or many yellow sectors of increased growth rate, with abundance of conidia or conidiophores, and of a relatively smooth or non-crinkled morphology. Such are consequences that are very much adaptive to the new temperature situation or stress, especially in the long-term, from the standpoint of the evolution of new adaptive strains of Aspergillus in terms of new differentiation patterns. Moreover, the configurationally, partial duplications, controlling such adaptation, are in effect an adaptively responsive, complex mutator system on the chromosomal or karyotypic level, and a system which exhibits internal regulation, though environmentally sensitive or responsive, and one whose mutagenic behavior is a-sexually inheritable via conidia and sexually transmittable to a F1 generation via ascospores (Lieber, 1972, 1976b.) As can be seen, this mutator system derives from genomic re-organizations on the karyotypic level. Many types of mutator systems, environmentally sensitive, derive from past genetic re-organizations (Lieber, 1972, 1976b).
This situation with Aspergillus indicates that inner-controlled, internally-regulated, very frequent karyotypic change can nevertheless be induced or influenced by a physical stress, namely high temperature, in such a manner that such controlled karyotypic changes result in adaptive changes on the differentiation/morphological, phenotypic level. This would be an example of a karyotypic-based, adaptively responsive differentiation/morphological change to a physical stress, a situation that has not been demonstrated before. This is highly significant as it now demonstrates that morphological and differentiation patterns can be adaptively responsive to stress through a stress-induced or modulated mutagenesis involving genomic configurations, which are in effect developmental, controlling elements on the karyotypic level apparently regulating the excision and insertion of smaller transposition elements within the configurations. The physical stress influences, possibly via cytoplasmic and membrane distortions, the inner-controlled mutagenic interaction of the genomic configurations in such a way that the control becomes enhanced leading responsively (within one generation) to very frequent, karyotypic-based, controlled changes in differentiation and morphogenesis in fungal colonies. Such responsive enhancement on the karyotypic level enables effective adaptive changes on the phenotypic level, that is, on the organismal level. The resulting karytotypic alterations or variations have become co-extensive with the many yellow mutant-sectors within each of a large number olive green colonies, and thereby, coextensive with a new, adaptive pattern of differentiation and morphogenesis. This is in effect a responsively induced, new karyotypic analogue of an adaptive differentiation and morphogenesis within a short period. This coextensive karyotypic variation would also be like an induced, genetic variation within a population on a micro-scale.
III. An Adaptive Phenomenon Apparently Unique in the History of Such Investigations
In the history of investigations into adaptively or environmentally responsive mutagenesis, the adaptive phenomenon involving Aspergillus was not previously observed, especially the process whereby controlled, very frequent karyotypic change under and through physical stress can be manifested adaptively in a short period as very frequent, adaptive changes in morphology, growth, and patterns of differentiation within growing, multicellular fungal colonies under stress. It is appreciated, however, that such responsive adaptation via karyotypic-mutator systems, whether or not transposition elements are involved, may not be perfect, as some karyotypic changes could be deleterious. Nevertheless, the type of environmentally responsive mutator systems within Aspergillus could have themselves evolved into more effective mutator systems, with developmental features, leading to more effectively adaptive developmental or morphological solutions to various types of environmental and internally-related epigenetic stress.
This phenomenon brings to mind the phenomenon of the genetic assimilation of induced morphological changes involving stress in Drosophila, first discovered and investigated by C. H. Waddington in the 1950s (Waddington, 1953, 1956a, & 1959.) For example, when developing Drosophila embryos are subject to ether vapor stress-treatments or shocks during a certain period in their development, a portion of the Drosophila develop two thoraxes with two pairs of wings in adult flies. During each fly generation exposed to ether stress, developed bithorax flies were inbreed or crossed. When after a relatively small number of generations of such inbreeding under stress, a very large proportion of the subsequent progeny resulting from repeated inbreeding for the new morphology, when not subject to ether vapor shocks during embryogenesis, still developed the bithorax phenotype as adults. In response to stress, the new morphogenesis has become genetically inheritable or assimilated in a relatively short period in some manner. In other experiments, where inbreeding also involved a relatively small number of adult generations, other types of morphological changes, such as changes in wing morphology, eye morphology and in anal excretory papillae, were also inheritably assimilated or canalized when generations of their developing embryos were subject to other types of imposed environmental stresses, such as temperature shocks with regard to wing and eye development and intense salt treatments of embryo food with regard to papilla size.
Though it was not demonstrated that many of such responsive, genetically based (or canalized), environmentally responsive morphological changes were adaptive to the environmental stresses, the genetically assimilated increase in papilla size as a response to salt stress may, however, have allowed adaptation to the increased salinity in a relatively short period and to any future increases, as Waddington pointed out. It was not ascertained whether or not new mutations on the gene level were induced through the imposed environmental stresses during embryogenesis, though this possibility cannot be ruled out, and is worthy of further investigation. Also, these morphological changes might have enabled the development of less obvious, internal adaptive features in a complementarity with the evident, canalized morphological changes. This possibility would also be well worth investigating. In this connection, see Lieber, 2011 regarding enabling mutations.
More recent, stress-involved assimilation-experiments were performed with a black caterpillar species. Developing progeny of such were subject to heat shocks within each developing, caterpillar generation. As a consequence, green adults developed during each of 13 generations subject to heat shock. Subsequently, developing caterpillars eventually became inheritably green without heat shock after 13 generations through repeated inbreeding of green progeny caterpillars that had developed in each of those 13 generations (Suzuki and Nijhout, 2006.) As the authors point out, it is feasible that such inheritably acquired color via heat stress would be adaptive as an effective camouflage in a environment of green, leafy vegetation during the warm season, and thus evolutionally adaptive in a relatively very short period in the context of evolution.
It was Waddington who had pointed out the importance of genetic assimilation in morphological and pattern evolution. Such developmental, genetic assimilation of features at the organismal level during evolution could have involved some types of genomic change on the karyotypic level, which they and their effects could have become repeatedly combined through a relatively short period of inbreeding, thereby accounting for an adaptive assimilation during a relatively, very short period, enabling an accelerated evolution. (Relevantly, the adaptive, Aspergillus mutator-system was created through types of inbreeding involving reorganized chromosomes.) Regarding such genetic or inheritable assimilation of environmentally induced characters, a change in genomic organization is clearly suggested (Piaget, 1974.) Such inheritable assimilation of environmentally-induced morphological changes and less evident, enabled features, could have contributed to the rapid evolution of developmental systems in various organisms.
The role of karyotypic mutators in this cannot be ruled out. This becomes especially feasible in view of the following found with the Aspergillus mutator system: one can generate, through an a-sexual selection from an extremely high mutant-sector, colonial producer at high temperature, a group of colonies with a significantly, further-increased mean frequency of mutant sectors at high temperature compared to the mean mutant-sector frequency of another group of colonies at high temperature [Lieber, 1972]. This would certainly suggest a genetic assimilation of a further increased karyotypic mutator effect at high temperature, possibly involving the stabilization of an epigenetic change, itself stressful. And the high temperature stress would be mutagenic in the context of the inner-mutator process, in a way, a non-linear, epigenetic extension of the mutator process. Occurring in other situations, this could have affected the rate of morphological evolution itself.
IV. The Evolution of Developmental Systems Due to Responsive Genomic Changes
In this connection, a high, non-linear rate or burst of karyotypic evolution has been correlated with a high, non-linear rate of morphological evolution in mammals and in higher plants (Wilson et al., 1977.) This may have involved karyotypic mutator systems similar to those described in Aspergillus (Cherry et al., 1978). Furthermore, such karyotypic mutator systems might even have been mutagenically responsive to various environmental and internal pressures or stresses, such as extremes in temperature and pre-mature aging. The consequences of such might very well have been corresponding, nearly immediate morphological changes that were adaptive to the new stresses. The inner-directed changes or reorganizations on the karyotypic level of genetic architecture could have resulted in corresponding, sudden reorganizations of regulatory genetic networks, leading to the higher level morphological changes. This may very well have accounted for the high, non-linear rates of morphological evolution of the mammals and higher plants. And there is some evidence that "morphological evolution relies predominately on changes in the architecture of genetic regulatory networks" (Prud'homme et al., 2007). Wilson et al. in 1977 also postulated the necessary involvement of genetic regulatory regions in the morphological evolution of mammals and of higher plants.
Also relevant to a karyotypic-regulatory basis for morphological evolution, responsive to stress, frequent duplications of karyoptype leading to polyploidy and corresponding morphological changes during plant evolution have been shown to be associated with periods of environmental stress (Vanneste et al., 2014.) Polyploidy in plants and general karyotypic change have been very adaptive and have greatly contributed to plant speciation. It cannot be ruled out that such changes in ploidy or karyotype have had, or involved, a developmental, mutator effect, determining in a controlled, specific, and refined manner genomic changes on the karyotypic level. Such mutator systems could have had their origin in those very karyoptypic re-organizations. Relevantly, the creation of allotetraploids can create karyotypic re-organizations that lead to or determine further genomic changes: "Recent studies, mostly with plants, suggest that polyploidization can induce a flurry of genetic and epigenetic events that include DNA sequence elimination and gene silencing." (Pikaand, 2001). Such internally directed, further genomic change or re-organization could also define the degree of evolution. As long ago as 1940, the geneticist, Richard Goldschmidt, argued that evolution, especially macro-evolution, could have involved the responsive or directed generation of mutation on the karyotypic/chromosomal level of organization, ensuing in the sudden occurrence of organisms with new developmental, primary patterns (Goldschmidt, 1940.)
As also pointed out several years ago by the author, karyotypic mutator systems may have contributed to and may have themselves become part of the evolution of developmental systems in various organisms, and in so doing, determining the very rate or degree of such an evolution (Lieber, 1972, 1975, 1976b, 1998.), thereby enhancing the evolvability of developmental systems. It is feasible in view of the adaptive Aspergillus system that such developmental systems would have been the result of an adaptively or environmentally responsive and evolving mutator system. Such an evolving system would have been due to its own inner-controlled, responsive instability. A consequence of this would have been the evolution in various organisms of even more effective, mutator-based developmental systems, wherein inner-controlled, minute karyotypic changes would have occurred as features of ontogeny. Moreover, such an evolving and integrative mutator system involving the architecture of the karyotype would have determined the very inner-evolvability of the evolution of development in various organisms, including and especially in higher plants. In effect, the responsively evolving karyotypic-mutator-system would be the responsively evolving capacity to evolve developmental systems, the inner-evolving evolvability of evolution. Another avenue for evolution involving mutators might have entailed a modern version of pangenesis, proposed by the author in 1967. (See Mutation, Development and Evolution.)
V. Likely Consequences and Possibilities from the Evolution of Karyotypic Mutator Systems
Though originally occurring years ago, investigations of the fungus, Aspergillus nidulans, have nevertheless explicitly revealed, through further examination, a new type of environmentally responsive, adaptive mutation of high degree on the karyotypic/chromosonal level, manifested phenotypically as adaptive changes in growth, differentiation and morphology. This phenomenon exhibited a temporal control responsive to an environmental stress, and it appeared to enable a quick, adaptive response to a physical stress. That is, it occurs when a flexible or plastic accommodation to physical stress is necessary. By means of its timing, the phenomenon is adaptively developmental through different levels of organization, from karyotype to pattern differentiation and morphogenesis on the level of the organism. Its controlled temporality is a key adaptive feature of this responsive phenomenon. The capacity to generate this adaptively responsive phenomenon and the adaptive, developmental consequences or features are themselves inheritable. This phenomenon might also be indirectly related to other environmentally responsive changes in development that have temporal features and that become inheritable, such as genetic assimilation. In effect, this karyotypic-mutator system is a responsive regulatory system producing new karyotypic configuations and an associated hierarchy of genetic and epigenetic regulatory changes that ensue in a new adaptive morphology and pattern. There may be other, unknown types of environmentally responsive mutator-systems yet to be discovered, which have also played a significant role in the developmental evolution of organisms. Nevertheless, it is likely that many developmental and growth-pattern systems in plants and animals have evolved from a basic, known developmental, karyotypic-mutator system, such as the one discovered in Aspergillus. Such systems could involve, refined, somatic intra-chromosomal recombination. In fact, the process of deletion and transposition in the Aspegillus mutator system was proposed to involve specific, somatic intra-chromosomal recombination implicating heterochromatin (Lieber, 1972, 1976b).
In various invertebrate animals, controlled karyotypic changes, such as deletions of heterochromatin, do occur within somatic cells as opposed to germ cells, during development (Goday and Estaban, 2001; Beerman, 1966; Waddington, 1956b, p. 352). Such deletions or excisions may occur through intra-chromosomal recombination (Beerman, 1966). And in certain amphibians, development is known to involve the creation of inheritable, irreversible nuclear (or chromosomal) changes within somatic tissue (see Fischberg and Blackler, 1961), these changes possibly being deletions. During lymphocyte differentiation in mammals, there is a regulation of genomic rearrangement events in those cells (Alt et al., 1986). It is well known that very high frequency, genomic changes involving somatic hypermutation/intra-chromosomal recombination in developmental, immunological tissues (B lymphocytes) occur as a controlled, adaptive response to internal environmental stresses, such as bacteria and viruses or other foreign antigens (Teng and Papavasilion, 2007; Ziqiano et al., 2004; Mange and Mange, 1990). The developmental consequence is diverse antibody production, which is adaptive. In various plants, there are controlled changes in ploidy in different cells during development (Bino et al, 1993; Galbrath et al., 1991). In Nicotiana, controlled deletions of heterochromatin in somatic cells, possibly involving intra-chromosomal recombination, occur frequently during development, which results in color variegation of the flowers (Burns and Gerstel, 1967). In maize, some features of development are based on a transposition-insertion-deletion, controlling-element system, with many variations of such (McClintock, 1951, 1965). Dr. McClintock proposed that many other aspects of maize development could be so based, as well. As in Aspergillus, such a system in maize derived from a chromosomal or karyotypic reorganization Such a system in maize and its variations are temperature and age sensitive.
These developmental systems have characteristics suggesting their evolution from responsive, karyotypic-based mutators. It is likely that other developmental systems having occurred through the evolution of environmentally responsive, changing karyotypes and based on innerlly-controlled, refined genomic changes, controlling genetic expression, will be dimonstrated. Such a system of refined, controlled genomic changes could involve the excision of genetic regions, the transposition of such, and their re-insertion into other sections of the genome. It is not difficult to imagine the evolution of such a refined system from a karyotypic-based mutator system, where in such a refined system, genetic material is not lost in most cases, but excised, transposed, and re-inserted, with developmental effects on higher levels of organization. The earlier and intrim stages of such an evolution may be exemplified in many current organisms.
Hence, it is predicted that more and various karyotypic-based mutator-systems, responsively generating or leading to frequent adaptive, inheritable changes in differentiation and morphology within short periods, will be detected in various organisms. As with the Aspergillus system, these mutator-systems may form the basis for the future evolution of more complex and refined developmental and growth pattern systems, leading to more adaptive and, in many cases, productive organisms. This would include cultivated and nurtured plants used in agriculture and horticulture, but among the harmful, could include organisms that are pathogenic to such plants, as well. The likelihood that the environmentally responsive mutator systems in bacteria, Aspergillus and maize are genetically related through evolution (Lieber, 1998) makes this prediction even more likely. The developmental, Ac-Ds controlling-element system in maize is similar to the mutator system in Aspergillus. The adaptively responsive phenomenon exhibited by Aspergillus (once classified as a lower plant) strengthens the case for the widespread occurrence of various types of adaptively responsive mutagenesis in various organisms, and gives greater feasibility to the conclusions stemming from those earlier investigations of adaptively responsive mutagenesis. The developmental, adaptive system in Aspergillus makes it even more feasible that environmentally responsive, inheritable mutator systems of various types, especially those with developmental features, have played a significant role in a responsively accelerated, adaptive, developmental evolution, especially that of animals, plants, including the progenitors of cultivated crops and of pathogenic organisms.
In fact, what appears to be a variation of such predicted situations, as described above, was recently described in April, 2014. When a soil fungus pathogenic to rice was subject in one experiment to increasing copper concentrations, which increases are normally toxic to the fungus, and to temperature shocks in other experiments, significant genomic rearrangements occurred in response to both types of stresses via the agency of transposition elements or TEs (Chadha and Sharma, 2014.) With increasing concentrations of copper in the culture medium, the fungal colonies became resistant, and were able to grow, which was correlated with increased or frequent genomic change through the insertion of certain TEs. Moreover, increased copper resistance was associated with frequent color changes of the colonies from grey to white, the changes appearing as white sectors in photographs, and also judging from the photographs, morphological changes were also generated. As noted by the authors, colonies adapted to the highest copper concentration exhibited dense aerial hyphae. Those colonies were completely white. In earlier investigations by these authors, temperature shocks or stresses affected fungal growth and resulted in morphological transitions such as pigment changes and the production of aerial hyphae (Personal Communication.)
These responsive, frequent genomic changes to stress appeared to have occurred over a short period, as implied by the data. Under field conditions, where there are high concentrations of copper in the soil in which the fungus resides, and the soil is very warm due to a tropical environment, this fungus exhibits a high degree of genetic diversity or genetic rearrangements, "suggesting [according to the authors] that high copper content of soil and temperature stress are among the important environmental factors responsible for the high genetic diversity of the pathogen under field conditions." The further implication is that such adaptive, genetic diversity was responsively induced via TEs over a short period.
They state: "Whereas, extensive research over the last several decades has elucidated numerous molecular responses to stress, it is much less known how these translate into organismal–level responses." They suggest that environmentally responsive TEs reflect such a translation. Does the color and morphological change of the colonies with regard to copper concentration also reflect such a translation? Recall in this connection, that a process involving transposition elements may also have been involved in the adaptively responsive mutator situation in Aspergillus nidulans, where frequent adaptive changes involving color-pattern differentiation, growth and morphology were generated over a short period. In support of such involvement of controlling elements, transposition of genetic elements, thought to be tandem duplications, from chromosome to chromosome in Aspergillus nidulans induced morphological and pigment changes within short periods (Azevedo and Roper, 1970.) These transposing elements responsible for those phenotypic changes had their source in a duplication derived from Dp I. Whether or not such phenotypic changes, based on such small, mobile, karyotypic segments, were adaptive was, however, not investigated. Yet, studies by the author showed that high temperature could significantly increase, within a nine-day period, the frequency of generation of this genetically based phenotype (Lieber, 1972.)
The adaptive processes as reflected by internally regulated, frequent karyotypic change and environmentally responsive TEs may only be markers or shadows of a deeper, more encompassing adaptive dynamic, the elucidation of which may give better insight into the translation mentioned above. With this in mind, the following questions arise: How and why would the environmentally responsive and innerely-controlled karyotypic changes, mediated by TEs, develop into adaptive phenotypes? What are the underlying connections that translate environmental cues or stresses into adaptive, organismal, developmental responses, from phenome to genome and through genome to phenome? The authors of the 2014 publication regarding the pathogenic fungus do point out that the TEs investigated do behave in different ways and are highly specific; responding differently to different environmental clues or stresses. Again, what is the basis of such specificity of action leading to a correct phenotypic adaptation?
VI. Conclusion. Towards Strengthening the New Paradigm with Constructive Results
Though it appears karyotypic-mutator systems, through their own environmentally responsive, inner-controlled instability, could have adaptively evolved into many current developmental systems based upon inner-controlled genomic changes involving transposing genes, it is still not clear in many ways as to how specific adaptive changes on various levels could have been mediated during that evolution. In this regard, could a type of dynamic, epigenetic imprinting due to various stresses, via cellular states, cellular membranes, the cytoskeleton and nuclear matrix, on chromosomal behavior and architecture, be involved in such specifically responsive adaptations? And could such an imprinting account for a likely accelerated evolution of pathogenic organisms and higher plants through an epigenetic imprinting process regulating and determining lasting karyotypic mutator influences on the very developmentally-involved epigenesis? Most relevantly, and predictable in this regard, inheritable epigenetic modifications in plants occur due to environmental stresses (Boyko et al., 2011). Such inheritable, adaptive epigenetic modifications, which the authors refer to as epimutations, are associated with an increased frequency of genomic rearrangements, whose generation appears to be non-random.
Such a further evolved, environmentally responsive process could be considered as a transgenerational, environmentally responsive developmental system, perhaps a variation of genetic assimilation. It would be one manifesting and occurring through dynamic connections across different levels. As far as elucidating the dynamic underlying such specific connections and interconnected adaptations on various levels of organization, including the environmentally responsive, transgenerational epigenetic-karyotypic level, one must look for more interconnected, holistic and imaginative explanations, based on new assumptions. One such assumption could be external forces imprinting stable-specificity through instability within and between cellular epigenomes or architectures. These explanations could and should be tested by experiment in order to gain a more complete, empirically-based picture and so enable scientists to arrive at a heuristic, universal principle in biology.
Knowing such a principle may enable scientists to counter or reverse the generation and evolution of pathogenic organisms and promote the evolution of pathogenic resistance in crops, as well. Be this as it may, and pointing to aspects of such a principle, environmentally responsive and innerly-controlled, adaptively changing karyotypic-mutator systems, involving transposons, could have provided the inner dynamic and capacity for various, enhanced macro- and micro-evolutions of various organisms and their developmental processes over relatively short periods. Using tissue culture methods, the creation and application of such mutator systems in an epigenetic context, involving transmitted energies and stresses, may even become a significant parameter in a near-future evolution, through genetic engineering, of more productive and age-resistant plant-cultivars with altered, adaptive developmental and growth pattern systems. These would be developmental changes and features analogous to those generated by the mutator-system in Aspergillus. The Aspergillus-mutator-system is an early and significant example (effectively in 1972) of an internally regulated hypermutator-system in a multicellular organism enabling, quick adaptive responsiveness, on various levels of organization, to new environmentally-induced conditions in the organism, and thereby innerly and developmentally evolutionary. The Aspegillus-mutator-system can certainly be seen as being within an epigenetic system guiding, and being cyclically influenced by, inner mutator processes, and one most likely prone to inheritable imprinting.
This would be a type of mutator-based, multilevel epigenetic system probably forming the evolved basis for many, present day developmental and growth-pattern systems, at least significant features of such, where controlled genomic change through responsively regulated genetic deletion, transposition and re-insertion could be involved in many situations. Of course in many cases, regulated gene activation and suppression occur as features of development. Yet, such genetic behavior is dependent on chromosomal configurations or states, such as heterochromatin and methylation. And, predictably, these could very well be epigenetically controlled, and controlling, through the environmentally-influenced deletion, re-insertion and expression of genetic factors, such as transposons, a process representing a type of position effect variegation through regulated intra-chromosomal behavior. Modern genetic research has provided supportive evidence of this (Ito et al. 2016), giving further support to the predictions presented in this article.
As shown by Ito et al., an epigenetic system in a higher plant can induce enhanced, inheritable, and adaptive mutation, through transposon insertion, enabling seed germination in response to a chemical stress in the culture medium that inhibits such germination in culture. This is an evolved, mutator-based system controlling development across generations, in which, transposon activity in progeny must also be induced or enabled by heat treatment of the parental generation: Such prior heat treatment of the parent plant, and ensuing transposon activity within the parent, also enables responsive transposon activity in seed progeny; namely, the chemical-stress induction of beneficial mutations, through the stress-responsive insertions of transposons into specific genes within the seed-progeny, requires heat treatment of the parental generation. Thus, heat stress itself would seemingly be acting or being utilized in a potentating-mutagenic, epigenetically adaptive fashion across generations. However, an implicated, controlling methylation of the inserted transposons---where methylation is under the regulation of another genetic region within the system---can inhibit the expression of the adaptive mutations, ensuing in re-sensitivity to the chemical stress. Subsequent heat treatment reactivates the expression of the beneficial mutations, as well as the expression of adjacent genes, through demethylation of the inserted transposons. The regulated methylation could mask the effect of such mutant genes in vivo when conditions would require plant dormancy. Under such cold conditions, as their research implies, the effect of the mutant genes would be non-adaptive but adaptive under warm conditions. The chemical stress is in fact a plant hormone that induces dormancy under cold conditions. In view of this, the evolved epigenetic control of mutant induction and expression would quickly be able to accommodate plants to changing environmental conditions, allowing for and inhibiting development when respectively necessary, and in a heritable fashion. And as noted, induced karyotypic change can cause genetic deletions and gene silencing in plants (Pikaand, 2001), which could be adaptive. Even though all the adaptive dynamics across different levels of organization have not been clarified in various studies, the predicted systems or processes such as these can nevertheless be seen as also contributing to the beginning stages of a new paradigm for mutation and evolution.
A new paradigm encompassing mutation and evolution not only becomes creditable but very feasible. As viewed through this paradigm change, environmentally responsive, enhanced genetic mutation on various genomic levels of architecture can occur while defining or structuring levels of biological evolution so guided responsively via epigenesis by that mutation. This would be, through mutator-processes, an inner-regulated, responsively enhanced mutation to stresses. Thereby, this would have been a mutator-defined mutation controlling the rapid and responsively accelerated evolution of organismal, developmental capabilities and their expression. On a deeper level, the evolution of developmental and growth pattern systems would appear to have an inner, ordering, stabilizing dynamic or component capable of quickly accommodating adaptively to environmental and internally-related epigenetic stresses, which tend to destabilize, and which in this context are mutagenic. Thus, evolution itself would appear to be a stabilizing, trans-generational, evolving developmental process, countering destabilization via multilevel, mutator-controlled, responsive mutation, through space-time. This perspective would not only have significant implications for agricultural research, such as crop improvement, but could guide medical research, as well.
Michael M. Lieber, August, 2014, December, 2015. and May, 2016
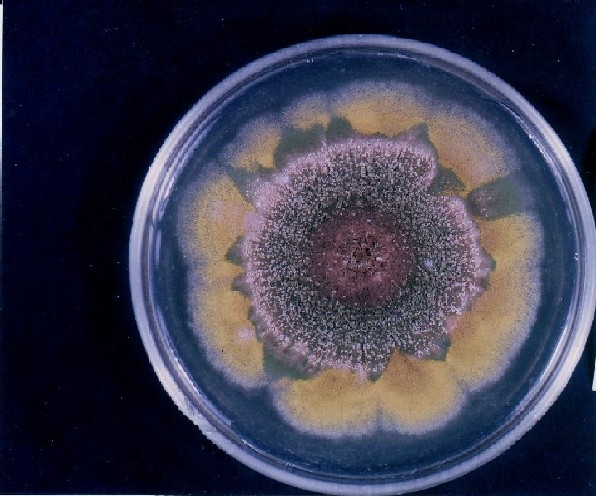
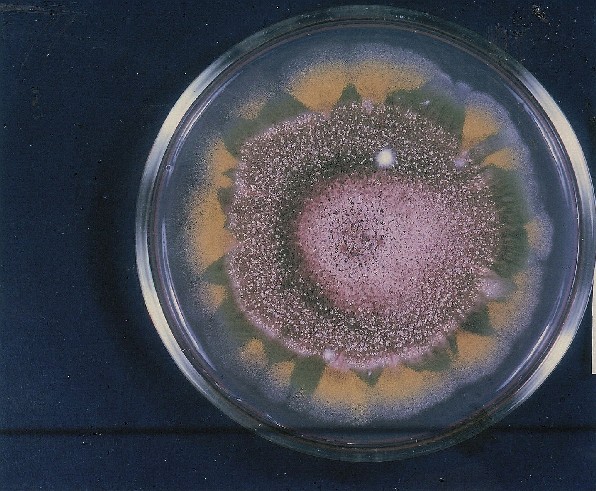
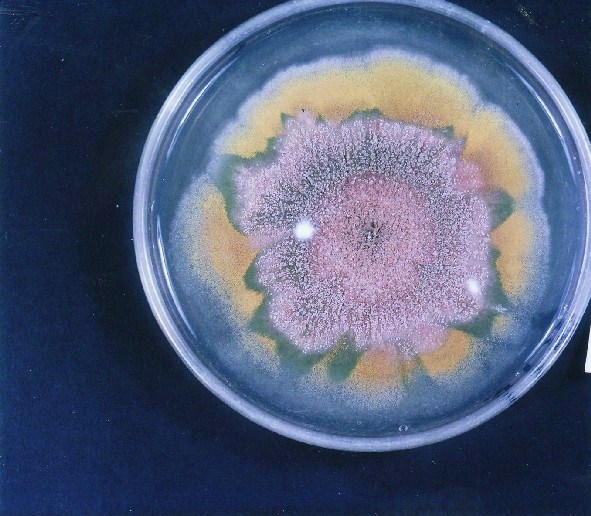
PHOTOGRAPHS OF ASPERGILLUS COLONIES
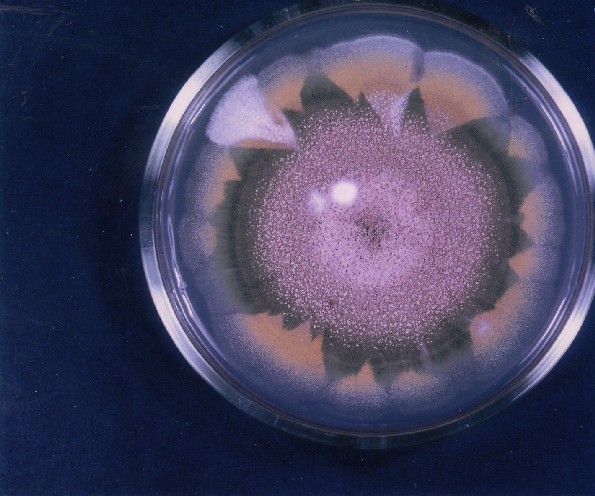
Colonies of Aspergillus nidulans from a large group of colonies each having produced through a karyotypic mutator system many mutant yellow sectors in response to a temperature stress. The improved morphology, growth-rate, and conidial production of such sectors would indicate an adaptively responsive, inner-directed mutagenesis to temperature stress. This would be an environmentally responsive hypermutation expressed phenotypically as a new pattern of differentiation encompassing morphological change within sectors. Also, note in the bottom photograph the two white sectors of normal morphology and improved growth rate. These arise due to mutations within a gene epistatically controlling the production of colored conidia. Such sectors were only generated, though infrequently, in the strain with the two partial duplications in the genome. Their generation in this situation might have been due to the insertion of a small genetic element from Dp III into (or very near to) the epistatic gene on chromosome II, resulting in the suppression of pigment production. Such insertion would have been in concurrence with deletions that would have otherwise produced yellow sectors.
References
Alt, F. W. et al. (1986). Regulation of genomic rearrangement events during lymphocyte differentiation. Immunol. 89: 5.
Azevedo, J. L. and J. A. Roper. (1970). Mitotic non-conformity in Aspergillus: successive and transposable genetic changes. Genet. Res. 16: 79-93.
Beerman, S. (1966). A quantitative study of chromatin diminution in embryonic mitoses of Cyclops fureifer. Genetics 54: 567-576.
Bino, R.J. et al. (1993). Flow cytometric detection of nuclear replication stages in seed tissue. Annals of Botany 72: 181-187.
Boyko, A. and Kovalchuk, I. (2011). Genome instability and epigenetic modification - heritable response to environmental stress. Current Opinion in Plant Biology 14 : 260-266.
Chadha, S. and M. Sharma. (2014). Transposable elements as stress adaptive capacitors induce genomic instability in fungal pathogen Magnaporthe oryzae. PLOS One 9, No. 4: 1-14.
Cherry, L., M. Lieber, and A. Wilson. (1978). Phylogenetic analysis of chromosomal evolution in vertebrates. A Report submitted to the Proceedings of National Academy of Sciences USA.
Fischberg, M. and Blackler, A.W. (1961). How cells specialize. Scientific American 205 (3): 124-132.
Galbraith, D. W. et al. (1991). System endopolyploidy in Arabidopsis thaliana. Plant Physiology 96: 985-989.
Goday, C. and Estaban, M.R. (2001) Chromosome elimination in sciarid flies. Bio Essays 23 (3): 242-250
Goldschmidt, R. (1940). The Material Basis of Evolution, Yale University Press.
Ito, H. et al. (2016). A stress-activated transposon in Arabidopsis induces transgenerational abscisic acid insensitivity. Published online in Scientific Reports 6. Article number 23181.
Lieber, M. (1967). Mutation, Development and Evolution. Thesis. Institute of Animal Genetics, University of Edinburgh.
Lieber, M. (1972). Environmental and genetic factors affecting instability at mitosis in Aspergillus nidulans. Ph.D. Thesis, University of Sheffield.
Lieber, M. (1975). Environmental and genetic factors affecting chromosomal instability at mitosis and the importance of chromosomal instability in the evolution of developmental systems. Evolution Theory 1: 97-104.
Lieber, M. (1976a). The effects of temperature on genetic instability in Aspergillus nidulans, Mutation Res. 34: 94-122.
Lieber, M. (1976b). The genetic instability and mutagenic interaction of chromosomal duplications present together in haploid strains of Aspergillus nidulans, Mutation Res. 37: 33-66.
Lieber, M. (1989). New developments on the generation of mutations in Escherichia coli lysogens. Acta Microbiologica Hungarica 36(4): 377-413.
Lieber, M. (1990). Mutagenesis as viewed from another perspective, Riv. Bio./B. Forum 83 (4): 513-522.
Lieber, M. (1998). Environmentally responsive mutator systems: toward a unifying perspective, Riv.Biol./B. Forum 91: 425-458.
Lieber, M. (2011. The problem of antibiotic resistant bacteria. The important role of environmentally responsive mutagenesis, its relevance to a new paradigm that may allow a solution. Theoretical Biology Forum. 104, No. 1: 91-102.
Lindegren, C. (1966). The Cold War in Biology, Planarian Press.
Mange, A. and E. Mange (1990). Genetics: Human Aspects, Sinauer Assocs. Sunderland, Mass.
McClintock, B. (1951). Chromosome organization and genic expression. Cold Spring Harbor Symp. Quant.Biol. 16: 13-47.
McClintock, B. (1965). The control of gene action in maize. Brookhaven Symp. Biol. 18: 162-203.
Piaget, J. (1974). Biology and Knowledge, University of Chicago Press.
Pikand, C.S. (2001). Genomic change and gene silencing in polyploids. Trends in Genetics. !7, No. 12: 675-677.
Prud'homme, B. et al., 2007). Emerging principles of regulatory evolution. Proceed. Nat. Acad. Sci. USA. 104, supp 1:8605-8612.
Suzuki, Y. and Nijhout, H.F. (2006). Evolution of a polyphenism by genetic accommodation. Science 311: 650-652.
Teng, G. and Papavasilion, N. (2007). Immunoglobin somatic hypermutation. Annual Review of Genetics 41: 107-120.
Vanneste, k., S. Maere, and Y. Van de Peer. (2014). Tangled up in two: a burst of genome duplications at the end of the Cretaceous and the consequences for plant evolution. Phil. Trans. R. Soc. B. 369, No. 1648 20130353.
Waddington, C. H. (1953). Genetic assimilation of an acquired character. Evolution 7: 118-126.
Waddington, C. H. (1956a). Genetic assimilation of the bithorax phenotype. Evolution 10: 1-13.
Waddington, C. H. (1956b). Principles of Embryology, p. 352. G. Allen and Unwin Ltd, London
Waddington, C. H. (1959). Canalization of development and genetic assimilation of acquired characters. Nature 183: 1654-1655.
Wilson, A.C. et al. (1977). Biochemical evolution. Annual Review of Biochemistry 46: 573-639.
Ziqiano, L. et al. (2004). The generation of antibody diversity through somatic hypermutation and class switch recombination. Genes and Development. 18: 1-11
Addendum
Though technically considered single-cellular organisms, bacteria and yeast grow into colonies of many cells. Shapiro (1988) argues that bacteria should be considered as multicellular organisms. Nevertheless, compared to Aspergillus nidulans, they would still be very simple multicellular, undifferentiated organisms. As noted, non-growing colonies of bacteria subject to a particular, nutritional stress produced mutations in single cells that gave rise to protruding, growing sectors of cell clones adapted to the nutritional stress. These sectors stain red while non-growing colonies from which they emerge are white. The production of such adaptive sectors under stress is very analogous, if not related, to the production of the mutant, adaptive yellow sectors in the colonies of the Aspergillus-mutator strain. This is especially so as the production of such adaptive sectors by the non-growing, stressed bacterial colonies was under internal, genetic control involving the excision of insertion elements from a regulatory gene (Hall, 1988).
This internal, genetic control responsive to environmental stress might reflect a very early form of developmental system displayed by the bacterial colonies. It would be a basic system producing adaptive sector-variegation or differentiation throughout the colonies by means of responsive, controlled genetic change. Each such colony with genetic-based, responsive, adaptive variegation might be considered as an adaptive, developmental whole or unit. This very early developmental system in bacteria might very well have evolved into developmental, karyotypic mutator-systems such as exhibited by the Aspergillus system and possibly in other, higher organisms, such as maize. In this regard, suggesting a developmental process, a two-part mutator system in bacteria, adaptively responsive to environmental, nutritional stress exhibited temporal control of the occurrence of adaptive mutations (Lieber, 1989, 2001). This temporal process involved transposons and recombination based on a mutant gene. In related studies with bacterial strains having a two-part mutator system involving transposons and recombination based on a mutant gene, directed, programmed mutagenesis in eight different bacterial strains enabled the same high frequency of growing colonies (within a short period) of each strain on respective media lacking the same group of amino acids for which the respective strains were genetically auxotrophic. This was in contrast to the response of auxotrophic, non-mutator strains. In effect (and implicitly), this represented adaptively responsive, directed mutation uniformly connected to overcoming the same multiple nutritional stresses within a short period, where transposition and recombination were involved (Lieber, 1989, e.g., pages 399, 391 and 385; Lieber.1998). Compared to the frequency of spontaneous mutations that would enable growth to the same group of amino acids under non-selective, non stressful conditions, the frequency of mutations conferring adaptation under the nutritional stresses would be exceedingly high. Responsive, developmental features were clearly indicated in this type of mutagenesis.
In this regard, each growing colony could represent an outcome of a subpopulation of stressed cells, which, through hypermutability of their genomes linked to the nutritional stresses, became adaptive to the stresses. These growing colonies would represent variegation within a sea or manifold of stressed, non-growing cells. This manifold with adaptive, growing variegations might be likened to a macro-colony undergoing inner-controlled, adaptive variegation or differentiation. This would make such a macro- colony a developmentally responsive entity, in many ways related to the adaptively responsive Aspergillus colonies through their environmentally responsive variegation/differentiation. This might suggest deep evolutionary, regulatory connections between bacteria and the fungi.
Very simple colonial organisms have, it would appear, inner mutator-systems capable of evolving into complex, environmentally responsive mutator-systems in relatively higher organisms, such as in Aspergillus and maize (See Lieber, 1998). Environmentally responsive mutator-systems with developmental features of various complexities appear to have been pervasive throughout the evolution of life through their own evolution. The evolution of such developmental mutator-systems from earlier, simpler ones to more complex ones would have given a further inner, evolving evolvability to evolution.
References
Hall, B. (1988). Adaptive evolution that requires multiple spontaneous mutations. I. Mutations involving an insertion sequence. Genetics 126: 5-16.
Lieber, M. (1989). New developments on the generation of mutations in Escherichia coli lysogens. Acta Microbiologica Hungarica 36(4): 377-413.
Lieber, M. (1998). Environmentally responsive mutator systems: toward a unifying perspective, Riv.Biol./B. Forum 91: 425-458.
Lieber, M. (2001). Temporal control of environmentally responsive hypermutation involving cryptic genes. Mutation Research 473: 255-257.
Shapiro, J. (1988). Bacteria as multicellular organisms. Scientific America. June: 82-89.